Product/Technology
Stent manufacturing processing
USP
・ Stents using laser processing, electrolytic polishing, etc.
・ Highly reliable medical equipment with various measuring instruments (radial hose measuring instrument, fully automatic 2.5-dimensional shape measuring instrument) necessary for stent manufacturing
・ Reliable supply of products in accordance with the ISO 13485 standard
Strength
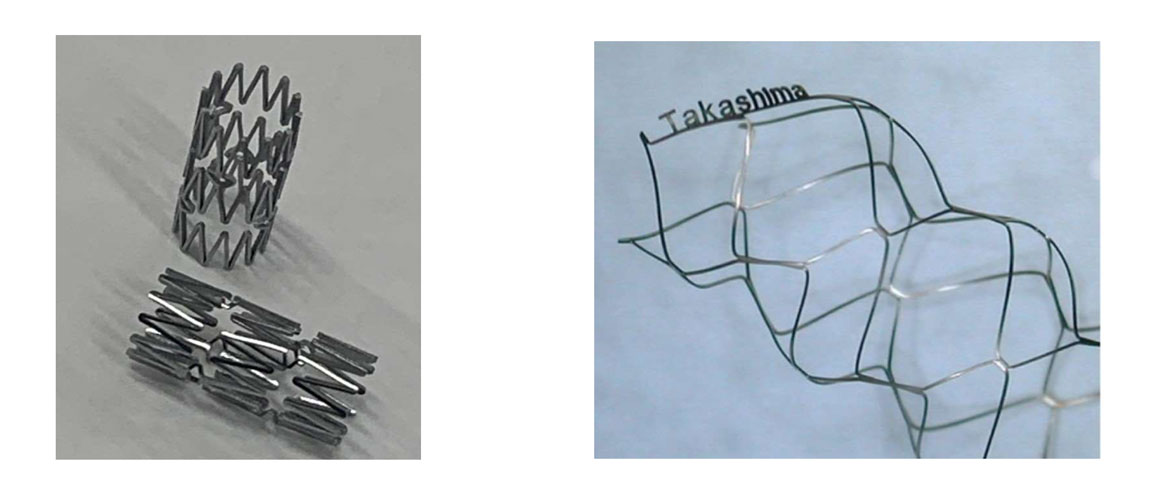
・ Stent processing using special alloys such as NiTi alloy and CoCr alloy.
・ In-house integrated processing of all processes such as laser processing and electrolytic polishing.
・ Functions are guaranteed using characteristic inspection equipment such as shape memory alloys.
Company
Takashima Sangyo Co., Ltd.
Address
5695-6 Kanazawa, Chino-shi, Nagano-ken
391-0012 Japan
Certification
ISO: 9001.14001.13485
PDMA (Class): Class I, II, and III
Certified STD. (Overseas): None
Notes
None