Product/Technology
Development of ODM products and module
USP
・ New medical product development implemented in concert with a customer in order to
untangle issues he or she faces, together with pursuing advancement in medical devices
enabling to improve patients QOL
・ Through study on in-depth needs while closely collaborating with our customers to exceed
their expectations
Strength
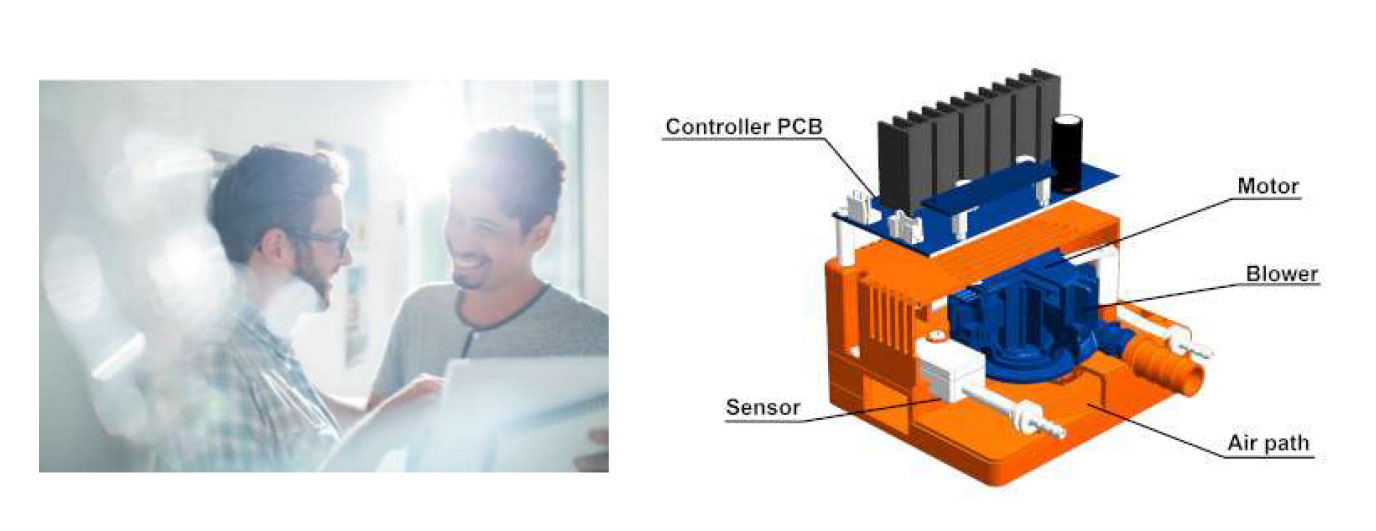
□ Development of motor and its peripheral module
□ Comprehensive support, until the launch of customer's products
□ Further expansion of global operations w/ new corporate brand called "ASPINA"
Company
ASPINA Shinano Kenshi Co., Ltd.
Address
1078 Kamimaruko, Ueda-shi, Nagano-ken
386-0498 Japan
Certification
ISO : 9001, 14001, 13485
PDMA (Class): None
Certified STD. (Overseas): None
Notes
ISO13485: 2016 (Production in Japan)
1. Manufacture of blowers for active medical
devices
2. Design and manufacture of compressors
for active medical devices